Ongoing Projects
Ongoing Projects

Alessia Beretta
Unit: Molecular Pharmacology
Director of Studies: Dr. Zaffaroni Nadia
Supervisors: Dr. Perego Paola
Genomic Characterization and Preclinical Model Development to Expand the Therapeutic Options for Epithelioid Hemangioendothelioma
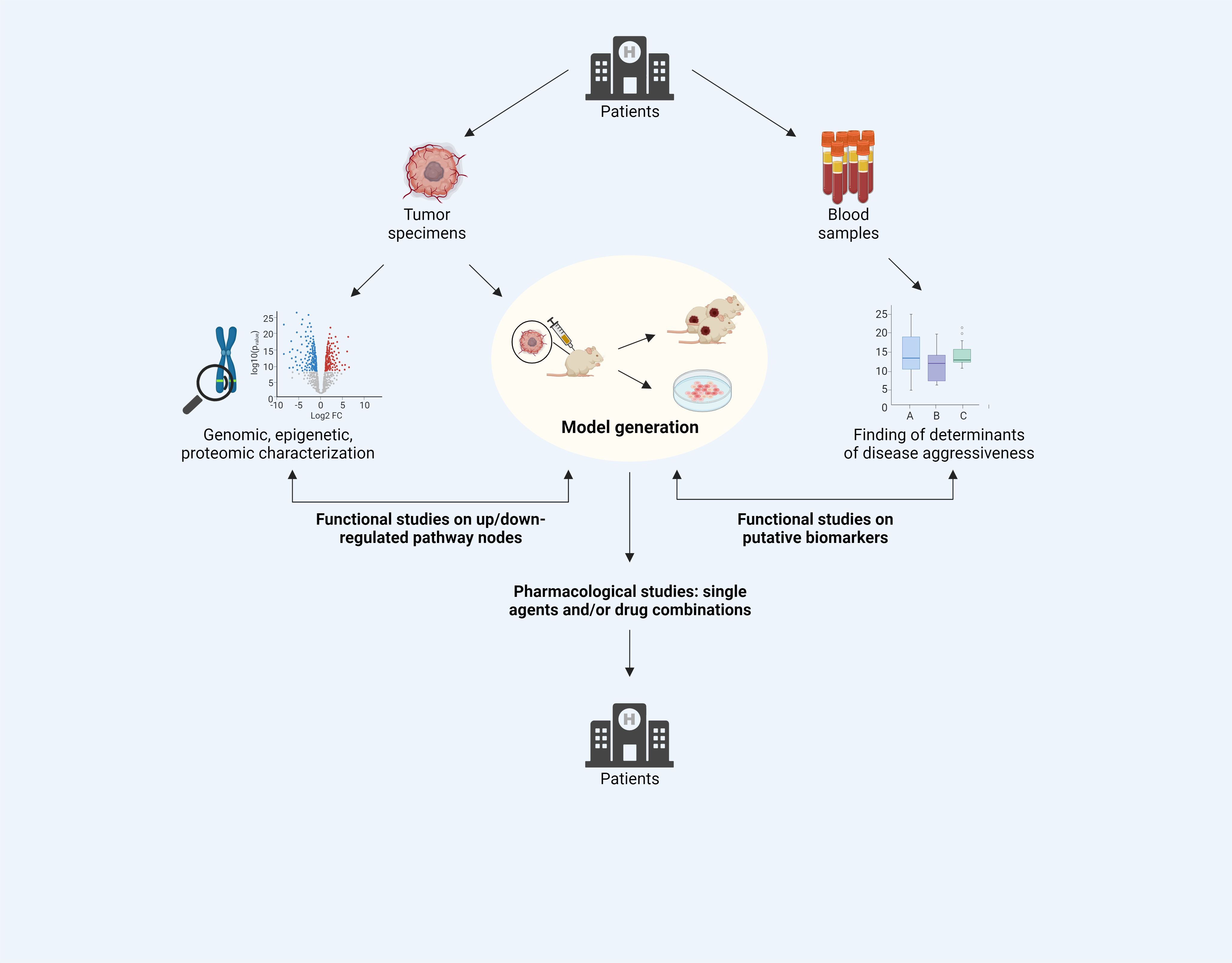
Epithelioid hemangioendothelioma (EHE) is an ultra-rare, translocated vascular sarcoma characterized by a heterogeneous and not predictable clinical behavior. No standard treatments are available and the response to conventional chemotherapy is extremely poor. This PhD project is part of a more extended project aimed at performing a genomic, epigenetic and proteomic characterization of prospectively collected EHE samples to identify novel biomarkers and new therapeutic targets. More specifically, the project focuses on the generation of EHE translatable models to use for molecularly characterizing this poorly understood disease and for validating novel therapeutic targets, putative biomarkers and determinants of drug resistance.

Giancarla Bernardo
Unit: Microenvironment and Biomarkers of Solid Tumors
Director of Studies: Dr. Sfondrini Lucia
Supervisors: Dr. Jachetti Elena
Targeting tumor-associated microbiota: a new strategy to hit tumor cells
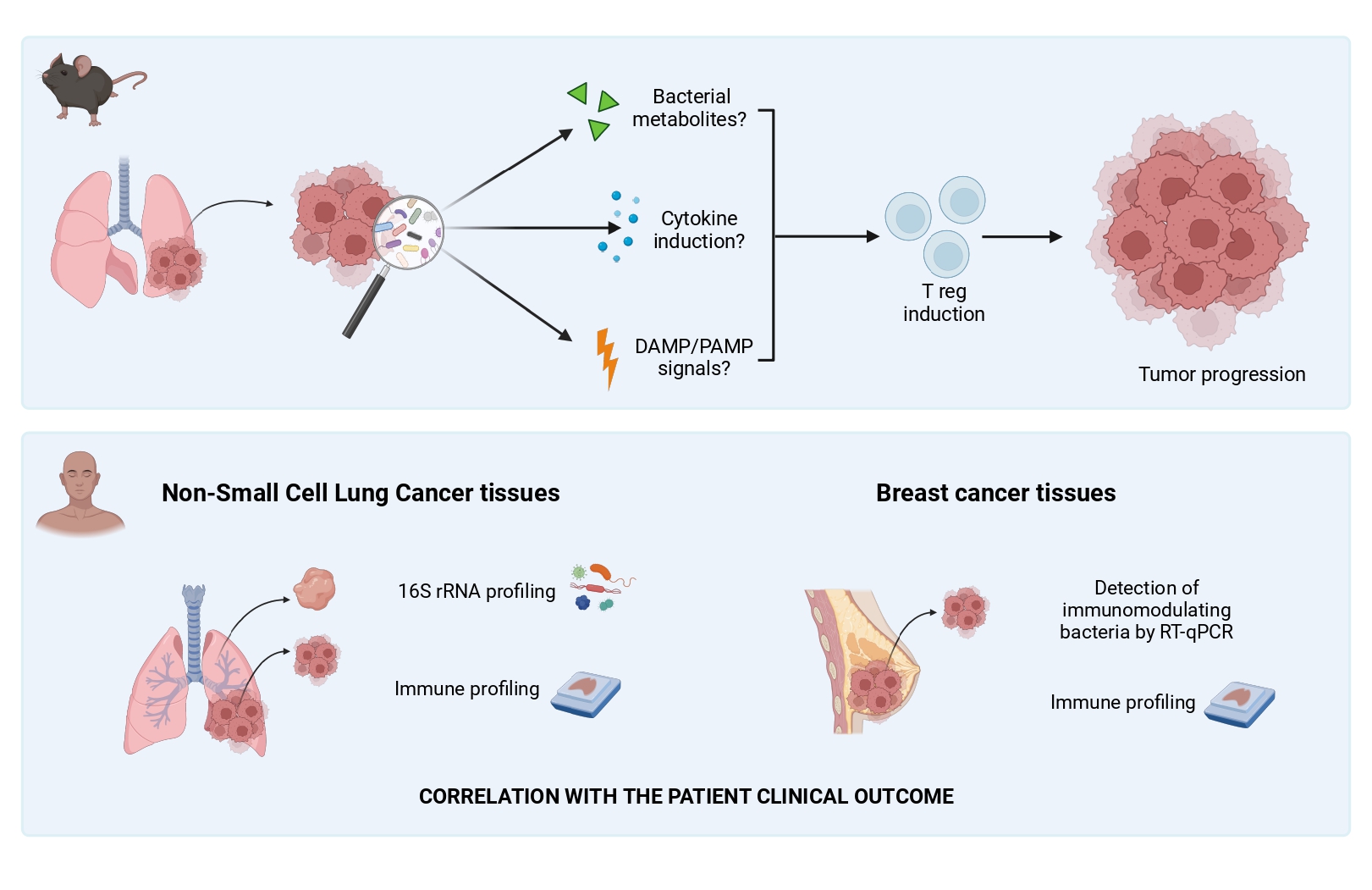
Emerging evidence has revealed that several organs and tissues once considered sterile, as lung and breast, harbor a unique microbiota. Recently, it has been reported that the tumor modifies the local microbial composition as compared to the healthy tissue, and that tumor-associated microbiota is involved in many pro-tumoral functions. In my PhD project, I am studying the impact of pulmonary microbiota on the growth of different murine model metastases, focusing on its effect on local immunosuppression. Moreover, I will analyze the microbial composition of human lung and breast tumor tissues to evaluate whether it correlates with the patient clinical outcome.
Michela Bianchi
Unit: Research in Nutrition and Metabolomics
Director of Studies: Dr. Pasanisi Patrizia
Supervisors: Dr. Chiodoni Claudia
Metabolomics and penetrance of BRCA genes: role of circulating microbiota metabolites as disease modulators in women with BRCA1/2 mutation
Women with BRCA1/2 mutations face a high risk of breast (55%) and ovarian cancer (16–59%), but not all carriers develop cancer, suggesting environmental factors like diet and microbiota may modulate this risk. The gut microbiota ferments dietary fiber into short-chain fatty acids (SCFAs), which have anti-inflammatory and anti-tumor effects, while high-fat diets can increase harmful lipopolysaccharides (LPS) linked to inflammation and cancer progression. This project aims to investigate the role of circulating microbiota metabolites (SCFAs) and dysbiosis biomarkers (LPS/LBP) as modulators of cancer risk in BRCA mutation carriers. It focuses on a cohort of 416 BRCA mutation carriers from the COS 2 study, who have completed a Mediterranean dietary intervention trial.


Roberta Bongiorno
Unit: Molecular Immunology
Director of Studies: Dr. Lecis Daniele
Supervisors: Dr. Triulzi Tiziana
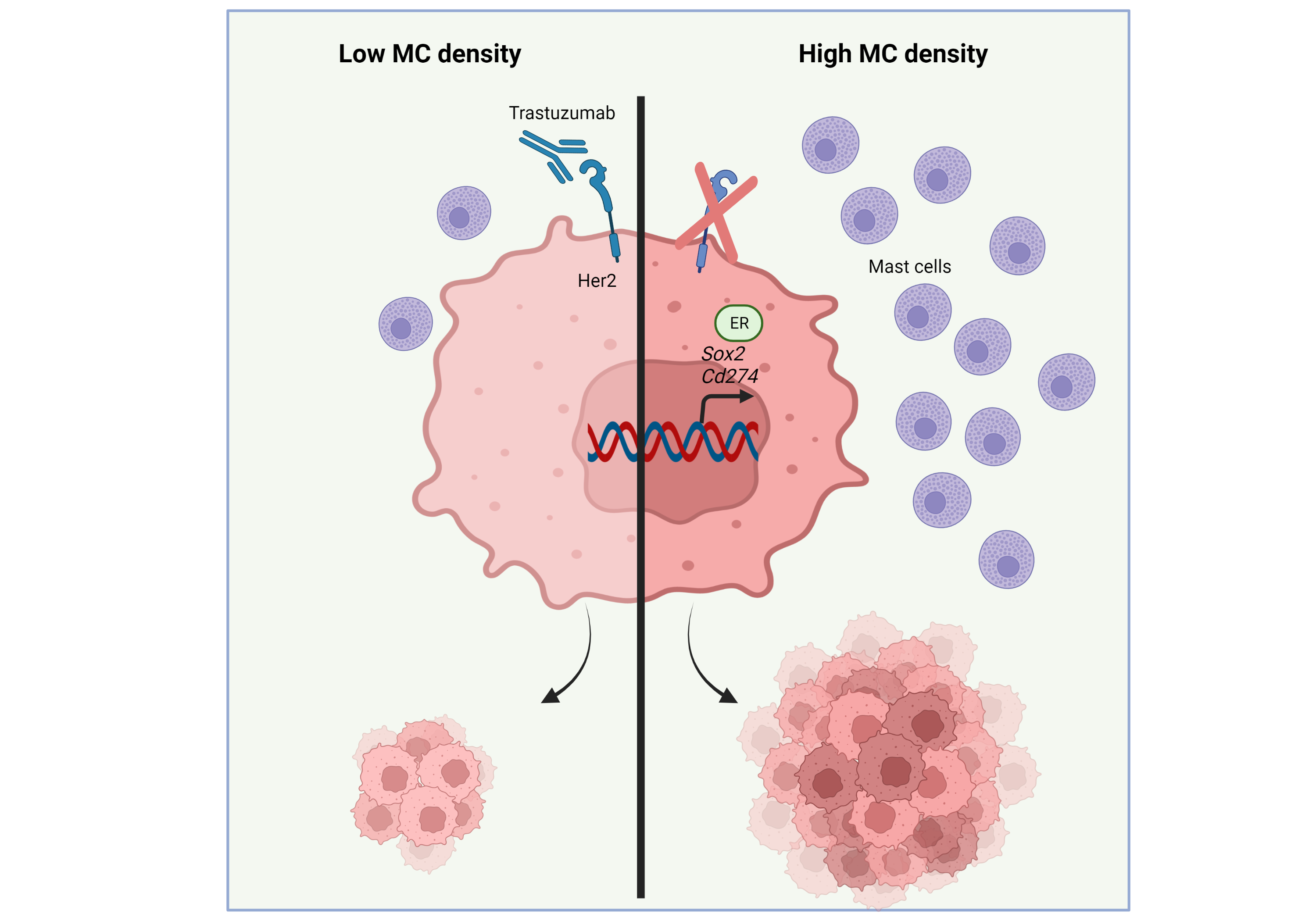
Dissecting the Role of Mast Cells in Breast Cancer Aggressiveness
Mast cells are immune cells displaying a controversial role in cancer. With my project, I aim to clarify their activity in breast cancer, with a particular focus on luminal and HER2 breast cancer, in which mast cells correlate with worse prognosis. I hypothesize that mast cells might affect breast cancer aggressiveness and therapy resistance through the induction of stem-like traits and the promotion of a luminal phenotype. The understanding of the molecular mechanisms through which mast cells affect disease outcome would improve the efficacy of treatment for patients characterized by high density of infiltrating mast cells.

Clorinda Brignola
Unit: Hereditary Digestive Tract Tumors
Director of Studies: Dr. Vitellaro Marco
Supervisors: Dr. Gariboldi Manuela
Improving the Identification of Hereditary Gastrointestinal Cancer: Paving Stones on the Road towards Personalized Precision Medicine
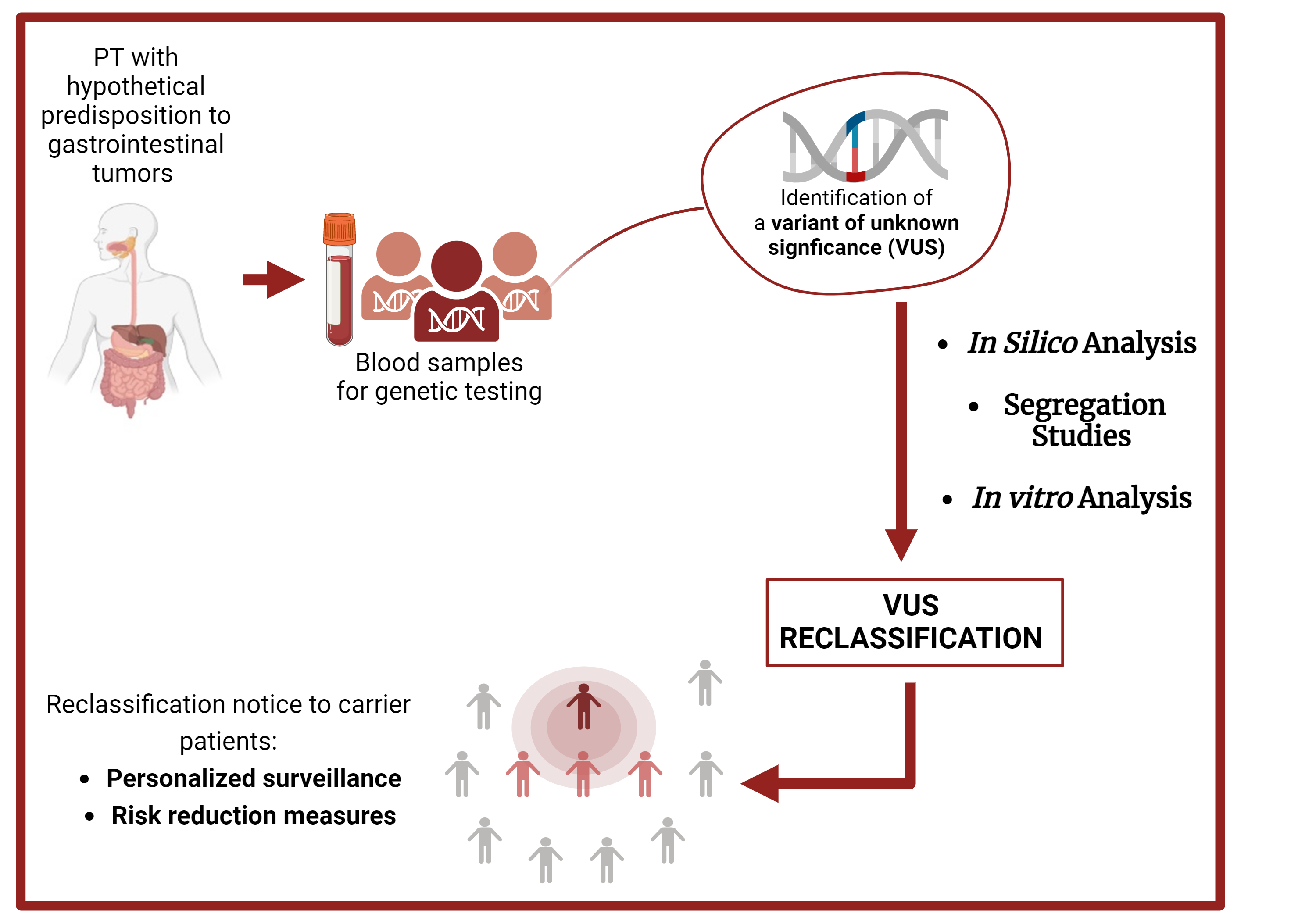
2-5% of gastrointestinal tumors are due to the presence of an hereditary predisposition mostly related to the presence of a constitutional pathogenic variant in a cancer predisposition gene. For some variants known as variants of unknown significance (VUS), it’s difficult to establish a clinical significance related with the risk of cancer occurence. In my PhD project I will focus on the reassessment of VUS identified in genes of hereditary predisposition to digestive tract tumors and their reclassification through in silico analysis, segregation studies and functional studies to better improve personalized surveillance and risk reduction measures.

Eleonora Citeroni
Unit: Integrated Biology of Rare Tumors
Director of Studies: Dr. Tomassetti Antonella
Supervisors: Dr. Bagnoli Marina
Exploring the involvement of choline kinase enzyme in ovarian cancer progression and dissemination
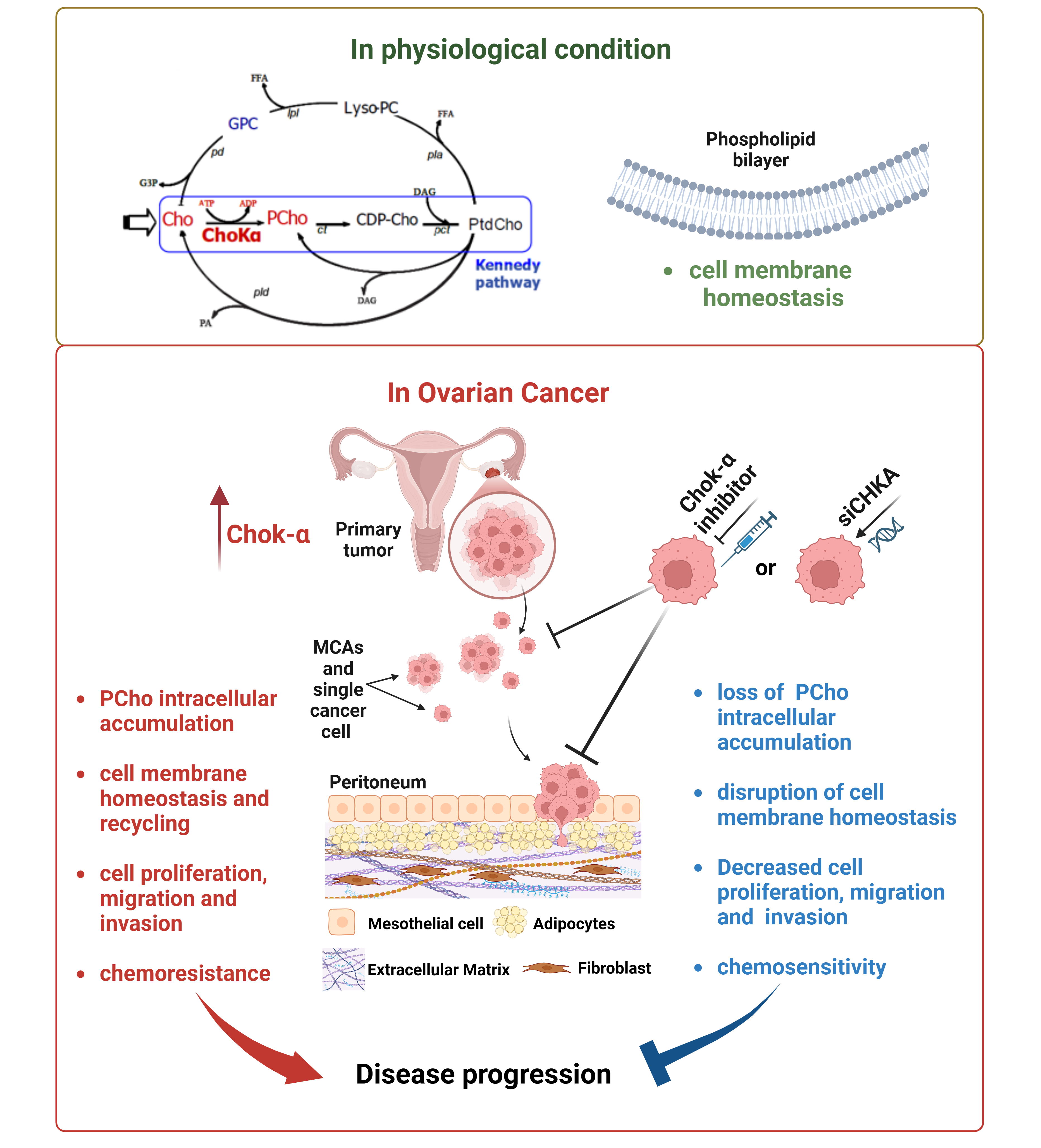
Epithelial Ovarian Cancer (EOC) is the deadliest gynecological cancer, with 70% being High-Grade Serous Ovarian Carcinomas (HGSOCs), which are highly heterogeneous and resistant to existing therapies. EOC cells show high levels of phospho-choline (PCho) and total choline compounds, due to the over-expression of Choline kinase alpha enzyme (ChoK-α). ChoK-α is the first enzyme of Kennedy pathway and catalyses the conversion of free choline in PCho, both a precursor and a breakdown product of phosphatidylcholine (PtdCho), a major component of cellular membrane. Our group previously showed that ChoK-α silencing reduces PCho intracellular content and OC cells aggressiveness, suggesting ChoK-α as a promising target. In my PhD project, I will explore ChoK-α's role in OC progression. By comparing CHKA silencing with a selective, first-in-class ATP-mimetic ChoK-α inhibitor, I aim to assess whether inhibiting ChoK-α's catalytic activity is key to induce disease regression.

Irene Fischetti
Unit: Molecular Immunology
Director of Studies: Dr. Jachetti Elena
Supervisors: Dr. Chiodoni Claudia, Dr. Mondino Anna (HSR, Milano)
Combinations of Local Radio- and Immuno-therapy to Induce Systemic (abscopal) Therapeutic Effects in Different Cancer Models
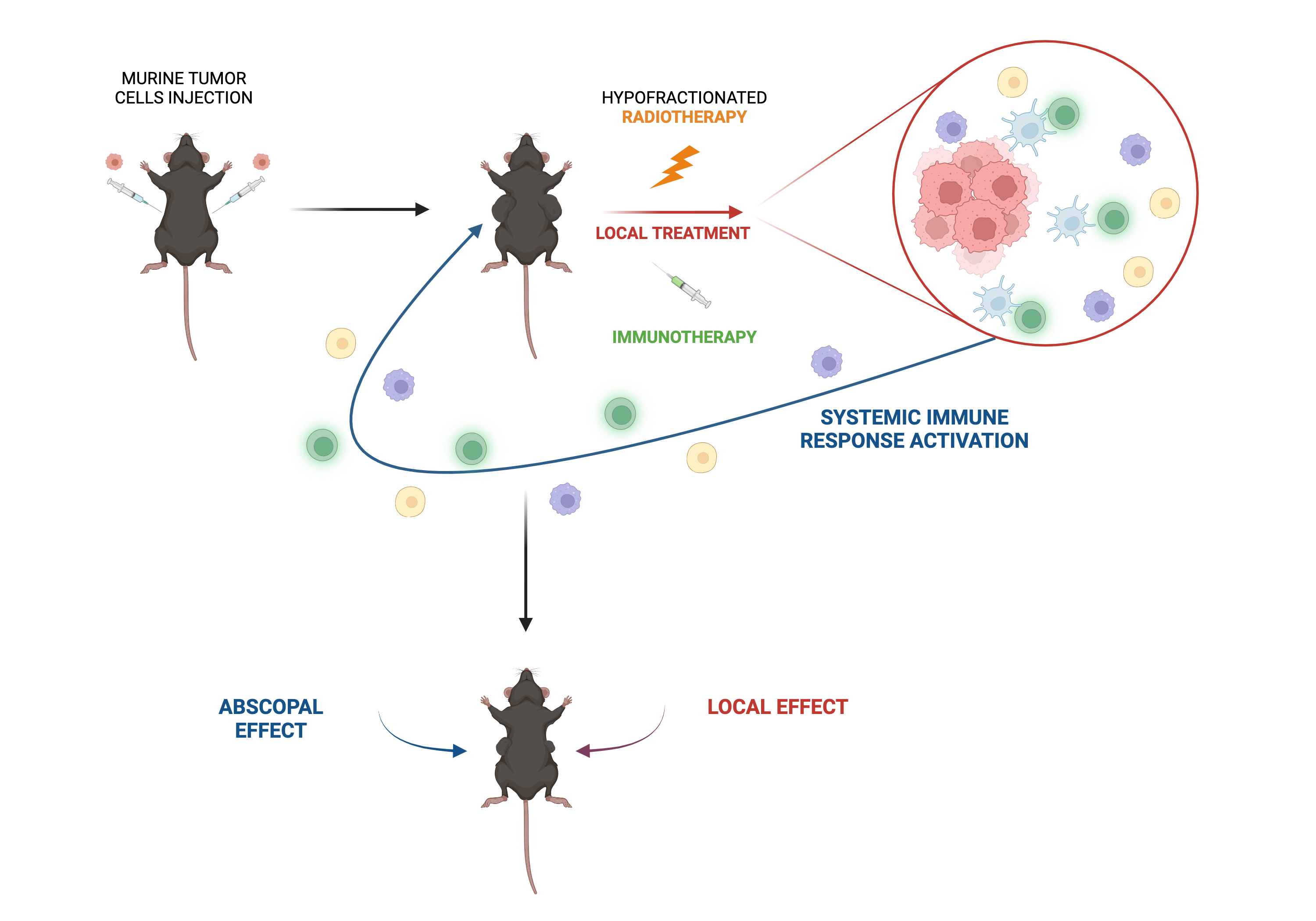
Radiotherapy induces DNA damage leading to apoptotic cell death. It can also exert both immuno-inhibitory and immuno-stimulatory functions, depending on tumour type and setting. Therefore, combining radiotherapy with immunotherapy has a great potential for cancer patients. Interestingly, in some instance, the activation of an anti-tumour immune response in one treated lesion can mediate the regression of distal metastases, not directly treated, effect called “abscopal”. The aim of my project is to evaluate, in different cancer models, the effect of combining radiotherapy with immunotherapy, locally administered within the tumour, in order to increase both local and systemic (abscopal) anti-tumour immune response.

Patrizia Ghidotti
Unit: Epigenomics and Biomarkers of Solid Tumors
Director of Studies: Dr. Fortunato Orazio
Supervisors: Dr. Jachetti Elena
Immunoregulatory role of Extracellular Vesicles in advanced Non Small Cell Lung Cancer
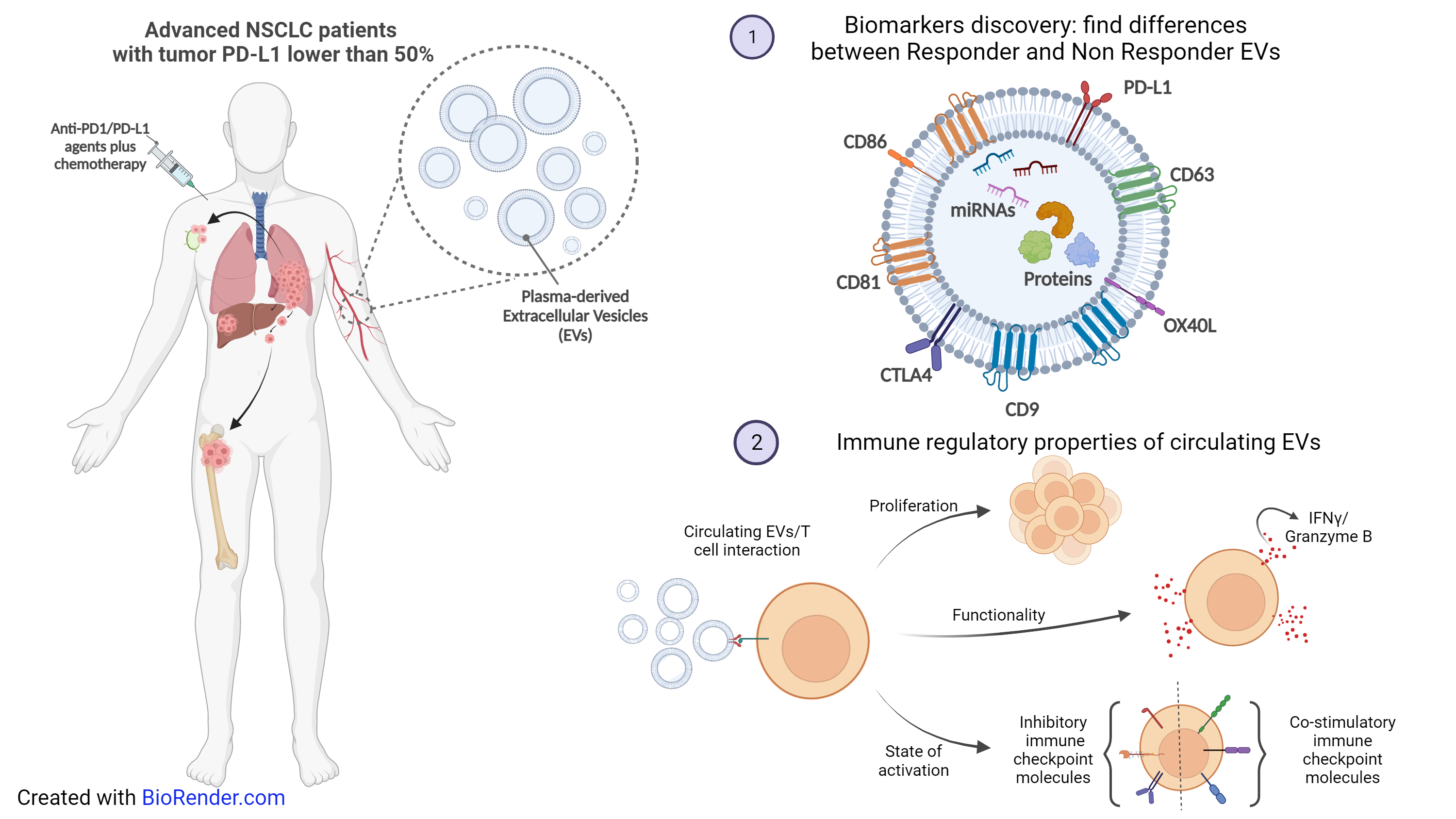
Immune-checkpoint inhibitors (ICIs) have improved clinical outcomes of patients with advanced Non-Small Cell Lung Cancer (NSCLC). Tumor PD-L1 remains the only clinical predictor biomarker of response to ICIs. Despite the efficacy of ICIs mainly correlates to high PD-L1 expression, also patients with low PD-L1 can benefit from ICIs. Extracellular vesicles (EVs) are membrane-limited particles described as biomarkers and immune mediators in cancer. In my project, I mean to evaluate plasma EVs of advanced low PD-L1 NSCLC patients in order to find biomarkers for combination therapy (chemotherapy plus ICIs) and to investigate their role as regulator of the anti-tumor immune response.

Giulia Guerra
Unit: Epidemiology and Prevention
Director of Studies: Dr. Venturelli Elisabetta
Supervisors: Dr. Huber Veronica
Application of a liquid chromatography–high resolution mass spectrometry metabolomics
Untargeted metabolomics analysis has emerged as a novel platform for studying cancer, enabling the detection of numerous metabolites to uncover mechanisms of cancer development and progression. However, standardizing and harmonizing data across diverse sources remains a challenge, complicating large-scale studies. Effective analytical design, including robust sample preparation and pre-analytical evaluations, is crucial for ensuring reproducibility, especially in studies involving thousands of samples.
My PhD project aims to establish a robust pipeline for HPLC-HRMS-based metabolomics tailored to cancer epidemiology. This involves optimizing sample preparation, refining analytical conditions, and developing stringent quality control protocols to ensure reproducible detection of different metabolite classes, enabling accurate and comprehensive metabolic profiling in prospective studies.
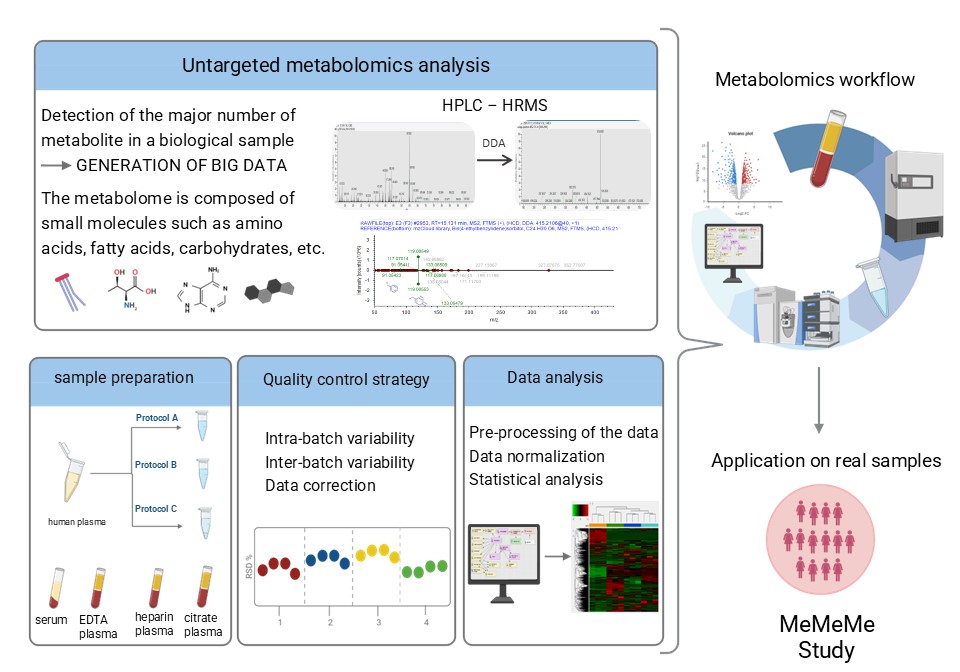

Luca Lalli
Unit: Translational Immunology
Director of Studies: Dr. Miceli Rosalba
Supervisors: Dr. Rivoltini Licia, Dr. Tosi Diego (Institut du Cancer de Montpellier)
Supervised and unsupervised pattern recognition analysis of immunological parameters in clinical setting
My project aims to improve the analysis of the huge and complex amount of immunological data obtained with high-throughput flow cytometry. I am developing deep machine learning methods, exploiting unsupervised clustering, to achieve fast and robust analysis by eliminating operator dependency. The main goal is to offer solutions for standardized manipulation of heterogeneous data, ensuring accurate comparisons between samples and simplifying the analysis workflow to save time and effort. This approach leads to a comprehensive understanding of the human immune response and allows the discovery of rare clusters and unknown patterns of immunological data. The expected result is improved clinical work-up due to an integrated, operator-independent analysis workflow.
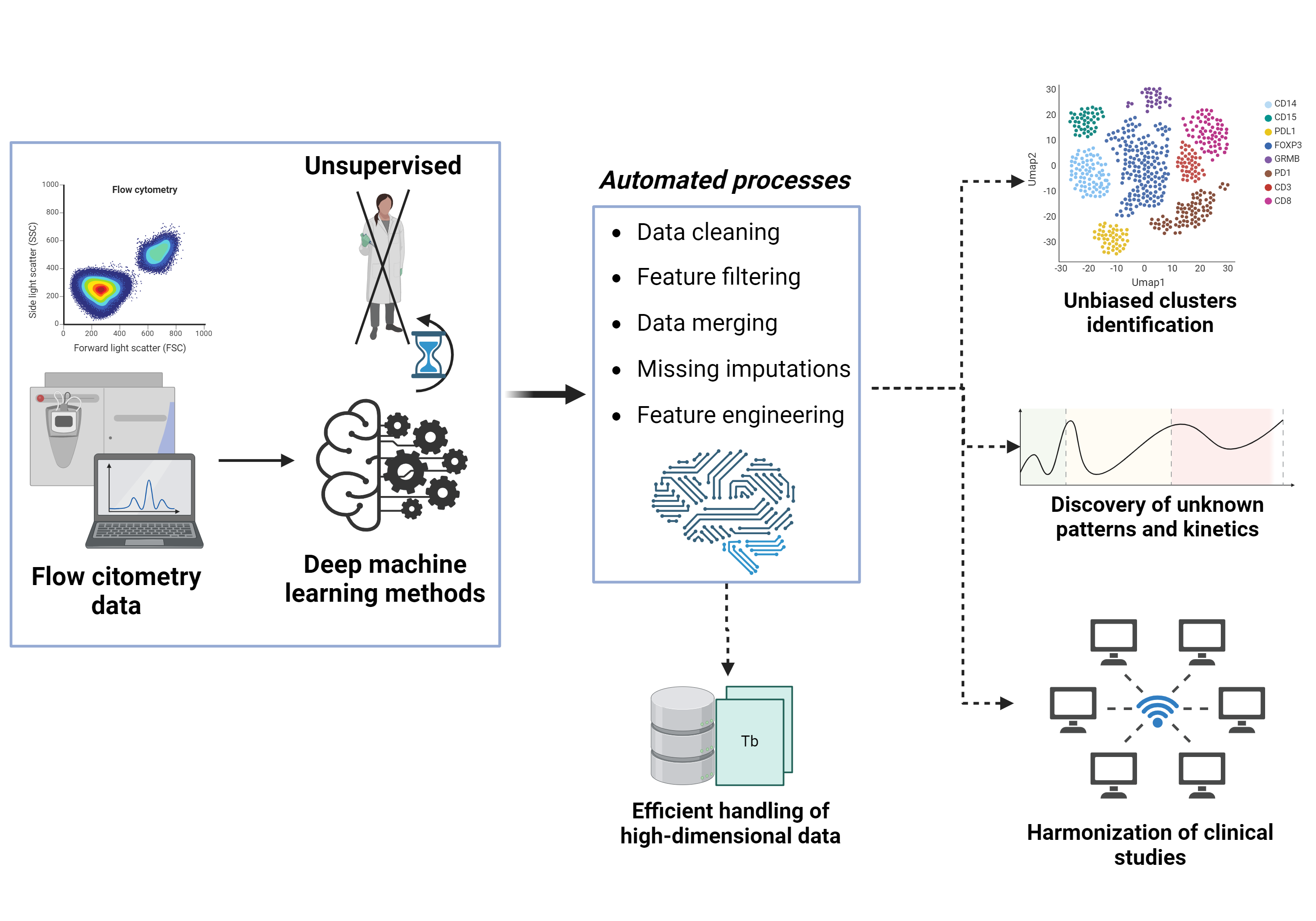
Armando Licata
Unit: Integrated Biology of Rare Tumors
Director of Studies: Dr. De Cecco Loris
Supervisors: Dr. Sfondrini Lucia, Dr Sozzi Gabriella
The role of microbiota in carcinogenesis and tumor progression in SSC
The role of the microbiome in cancer has gained recognition in recent years. In this project, my objective is to elucidate the microbiome's involvement in Squamous Cell Carcinoma (SCC). This project will use advanced sequencing and bioinformatics techniques to conduct a comprehensive exploration of the microbiome's relationship with SCC, focusing specifically on Lung Squamous Cell Carcinoma (LUSC) and Oral Squamous Cell Carcinoma (OSCC). Through ecological analyses, gene expression profiling, and cytokine assessments, I aim to establish the link between the composition of the microbiota and the progression of SCC. To accomplish this, I will employ a diverse set of omics approaches, including metagenomics, RNA-seq, and miRNA-seq, to delve deep into the microbial factors contributing to the development of SCC. The outcomes of this research have the potential to significantly enhance our understanding of SCC and inform the development of more precise and effective strategies for managing this type of cancer.
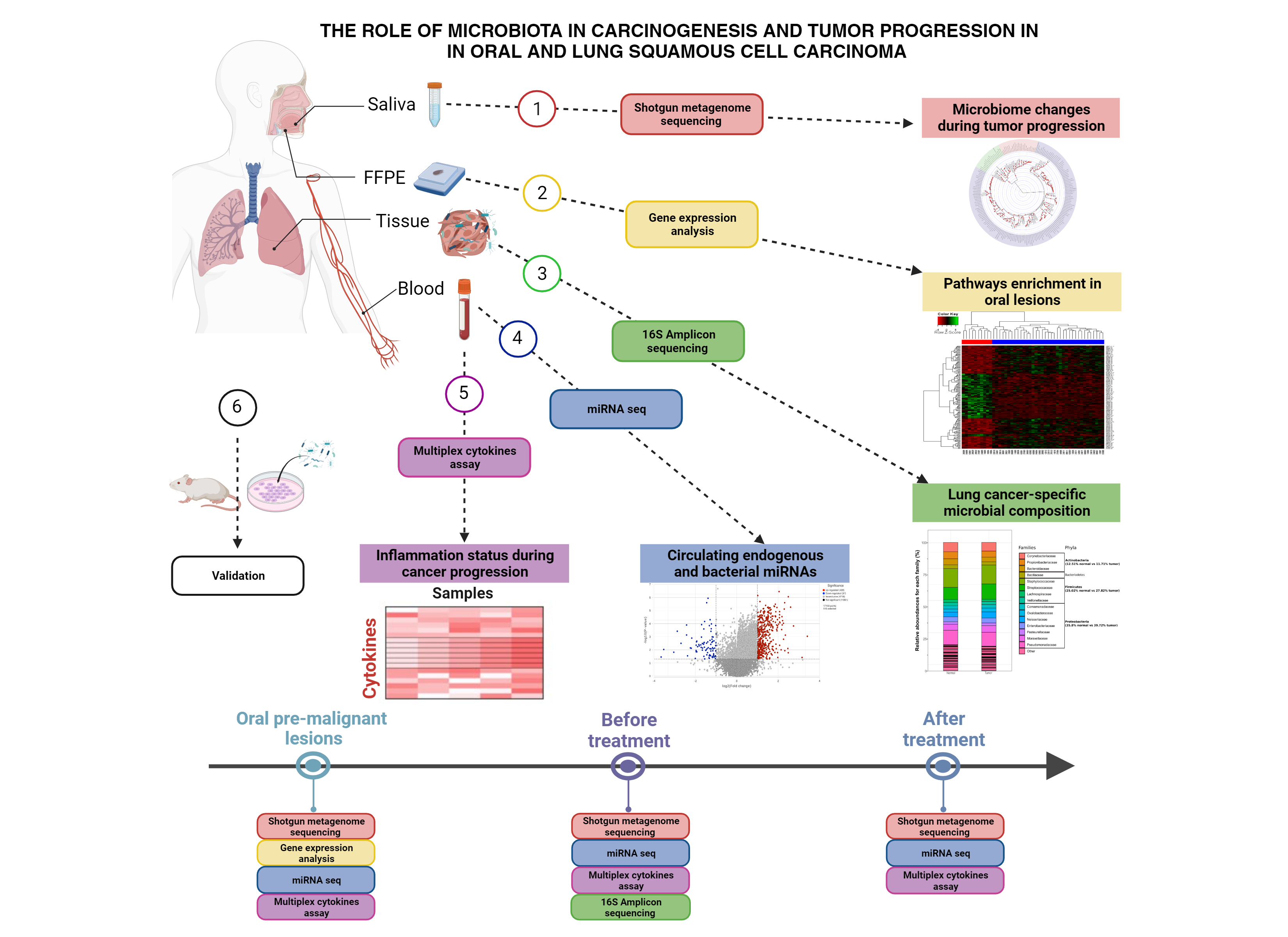
Arman Mandegar Khorasani
Unit: Molecular Immunology
Director of Studies: Dr. Sangaletti Sabina
Supervisors: Dr. Prelaj Arsela
Bone Marrow Mesenchymal Cell as sensor of early relapse in lung cancer
Despite advances in cancer treatments like targeted and immune therapies, lung cancer patients still face the lowest five-year survival rates. Improving survival outcomes, particularly through early detection of relapse or early-stage disease, is crucial. My PhD project aims to identify early relapse biomarkers by investigating the biological mechanisms driving relapse. Tumor development creates a continuous hematopoietic demand, primarily sensed by bone marrow mesenchymal stem cells (BM-MSCs), which then redirect hematopoietic stem cell (HSC) differentiation toward tumor-supporting immune populations. This "BM sensing" mechanism may occur early, preceding malignant transformation, and be marked by a peripheral enrichment of specific myeloid cell subsets cells.
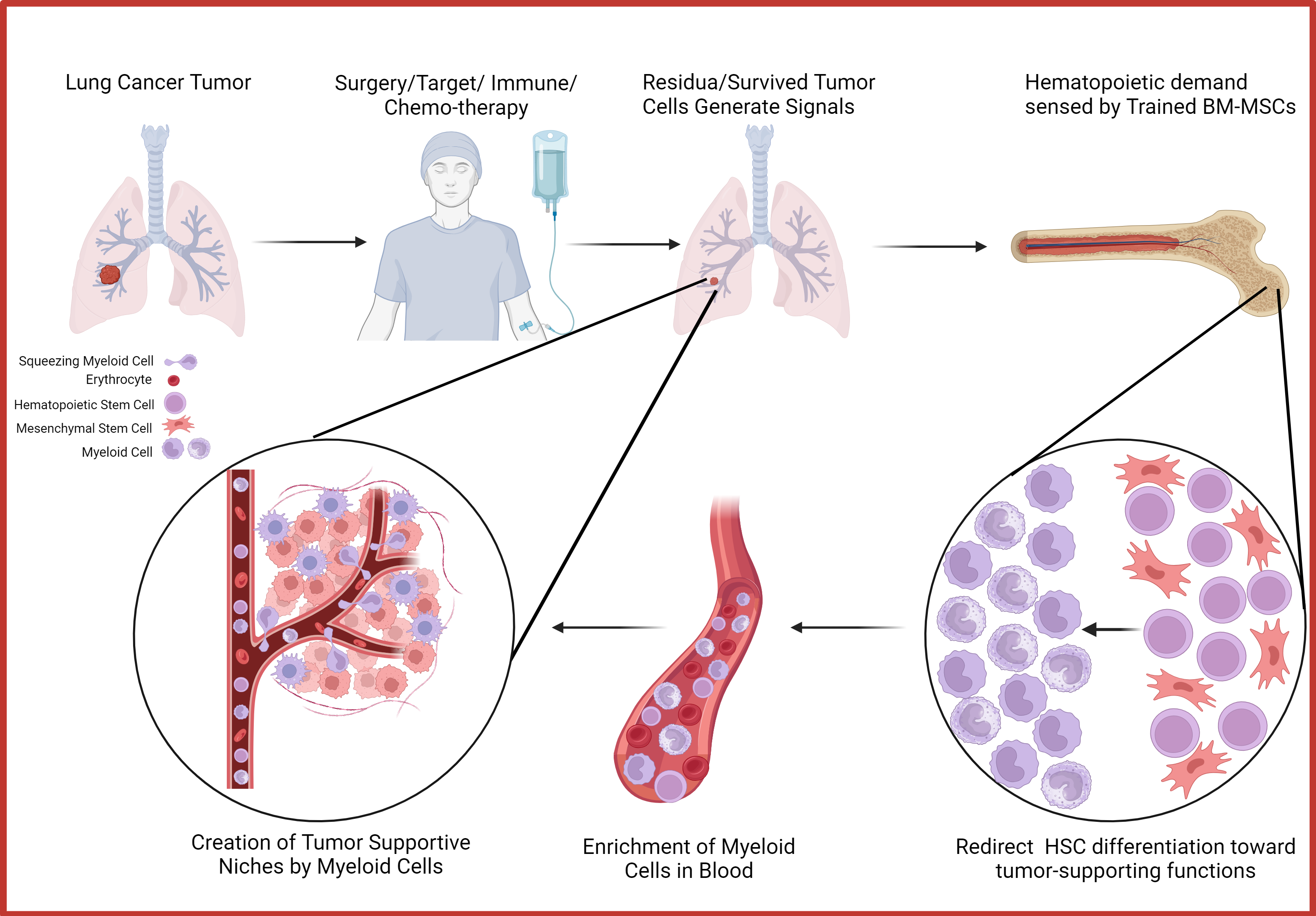

Beatrice Mazzoleni
Unit: Integrated Biology of Rare Tumors
Director of Studies: Dr. Greco Angela
Supervisors: Dr. Borrello Maria Grazia
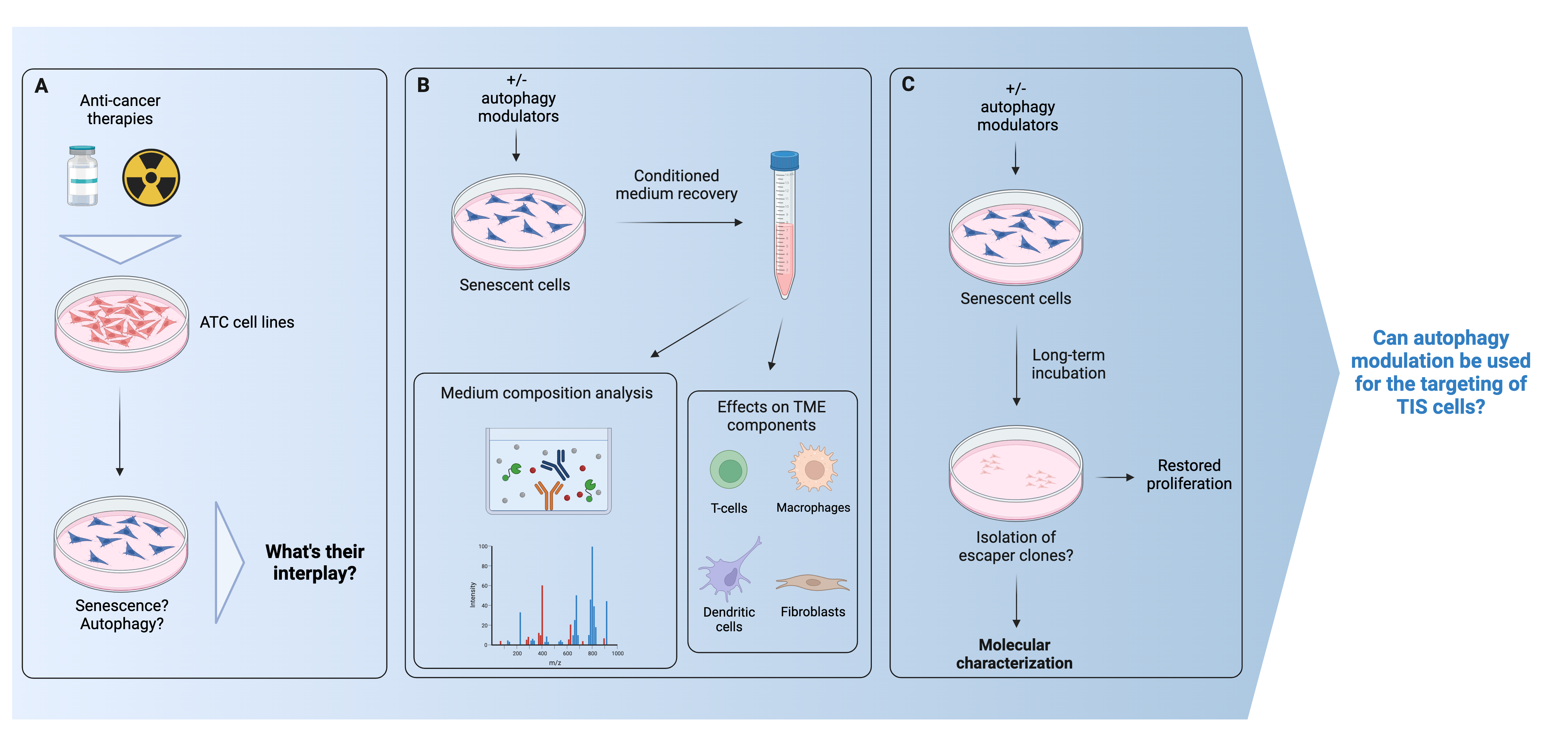
Role of autophagy in therapy-induced senescence in thyroid cancer
Anaplastic thyroid cancer (ATC) is among the deadliest solid tumors, for which no effective treatments are currently available. Several anti-cancer treatments induce senescence (therapy-induced senescence, TIS); unfortunately, TIS cells can fuel many aspects of cancer progression. With my project I aim to understand the role of TIS in ATC, and its interplay with autophagy, a catabolic process which is also often induced by anti-cancer treatments. The project might provide new insights into TIS cells biology, and unveil the possibility to target them by modulating the autophagic process.
Eisa Naghshineh
Unit: Molecular Pharmacology
Director of Studies: Dr. Folini Marco
Supervisors: Dr. Zaffaroni Nadia, Dr. Gandellini Paolo
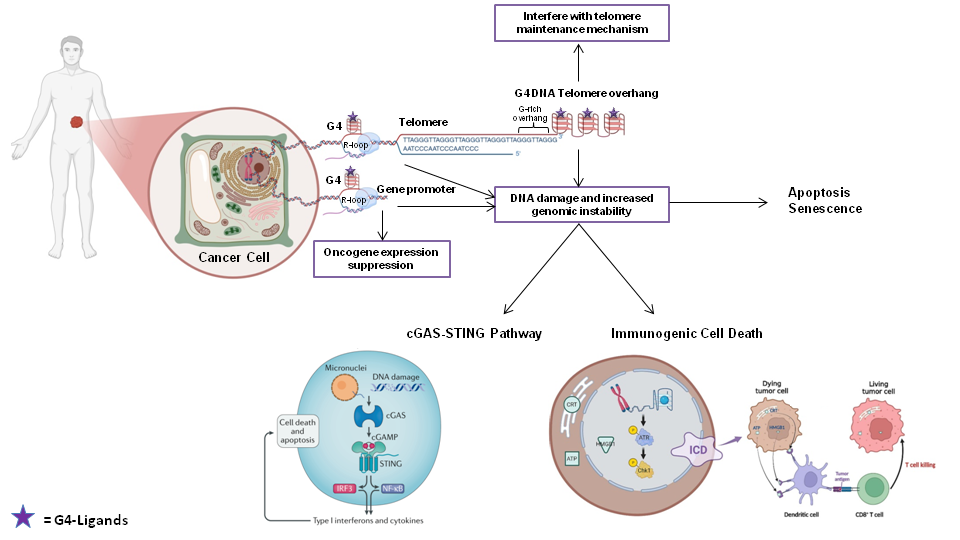
Harnessing the Biological effects of G-quadruplex-interacting Small Molecules for Therapeutic Purposes in Mesenchymal Tumors
Guanine-rich DNA sequences can adopt secondary structures called G-quadruplexes (G4), which are frequently found in oncogene promoters and telomeres. Several tumor-associated genes, the promoters of which may form G4, have been catalogued. These observations have provided the rationale to explore G4 targeting as a therapeutic approach. Several small molecules have been characterized as G4-ligands (G4-L); most show a “multiple-target” binding modality, which may be regarded as an advantage for developing multiple-hits, single-agent-based therapeutic approaches. My PhD project aims to 1) characterize at the cellular level the effects of small molecule-mediated stabilization of genomic and telomeric G4 structures in patient-derived soft tissue sarcoma cell lines; 2) identify common or tumor type-specific determinants of the response to G4-L that may represent additional therapeutic targets for the selected malignancies; and 3) explore the therapeutic potential of G4-L-induced DNA damage/genome instability related to the activation of immune gene response pathways in dedifferentiated liposarcoma (DDLPS) tumor cells.
Valeria Pinna
Unit: Molecular Immunology
Director of Studies: Dr. Jachetti Elena
Supervisors: Dr. Lecis Daniele; Dr. Crea Francesco (Open University, Milton Keynes, UK)
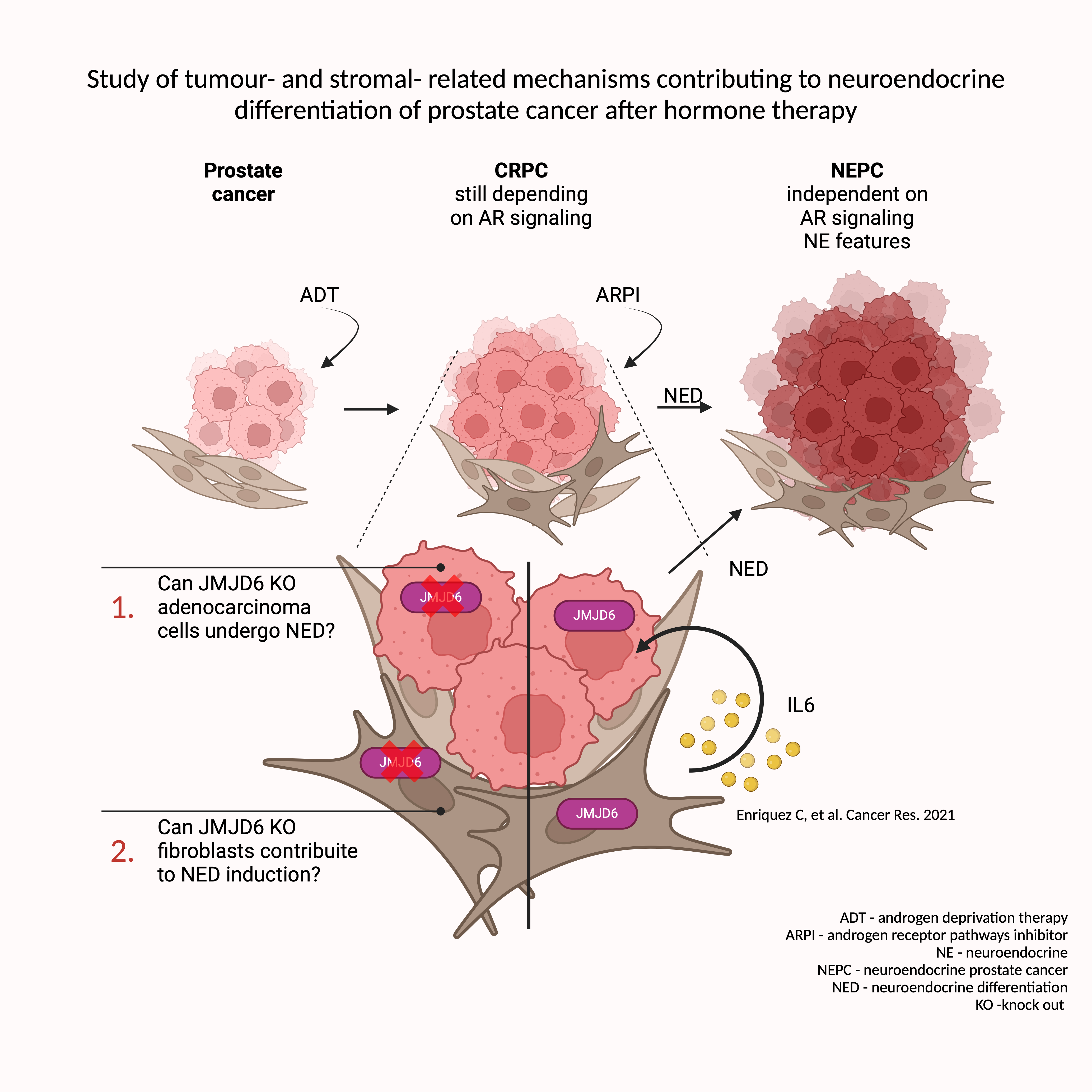
Study of tumour- and stromal- related mechanisms contributing to neuroendocrine differentiation of prostate cancer after hormone therapy
Castration resistant prostate cancer (CRPC) can undergo neuroendocrine differentiation (NED) to escape hormone therapy, becoming a more aggressive and uncurable tumour. NED is driven by epigenetic and stroma-derived factors, whose study is essential to define novel therapeutic targets. JMJD6 is an epigenetic regulator that can promote CRPC through the upregulation of the androgen receptor V7 variant. Since its contribution on CRPC progression, in my PhD project I want to investigate if JMJD6 could also impact NED. Moreover, I want to investigate the activity of JMJD6 on fibroblasts, components of the tumour microenvironment that we previously demonstrated can contribute to NED.
Francesca Putti
Unit: Medical Oncology 1
Director of studies: Dr. De Santis Francesca
Supervisors: Dr. Di Nicola Massimo
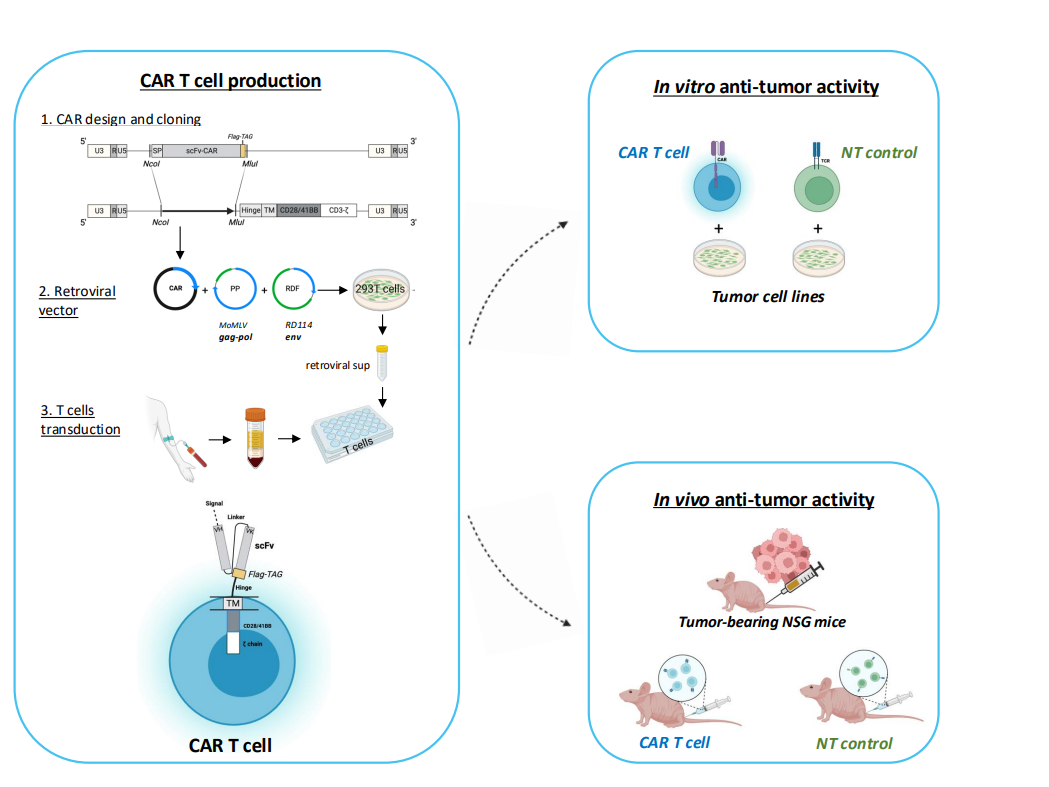
Design, development and optimization of novel CAR T cells using Globo H as a target for solid tumors cell therapy
Chimeric antigen receptor (CAR)-T cell therapy has been a clinical breakthrough in hematological malignancies; however, it is still facing multifactorial challenges for clinical success in solid tumor. For this reason, the aim of my research project is to design and manufacture novel CAR T cells for solid tumor cellular therapy. The project ought to re-direct T cells against a non-protein tumor-associated antigen that is overexpressed on several epithelial solid tumor, while absent on normal tissues. Once generated, novel CAR T cells will be tested both in vitro and in vivo for their anti-tumor activity and tumor-specificity.
Cesare Soffientini
Unit: Molecular Pharmacology
Director of studies: Dr. Zaffaroni Nadia
Supervisors: Dr. Folini Marco, Dr. Stacchiotti Silvia
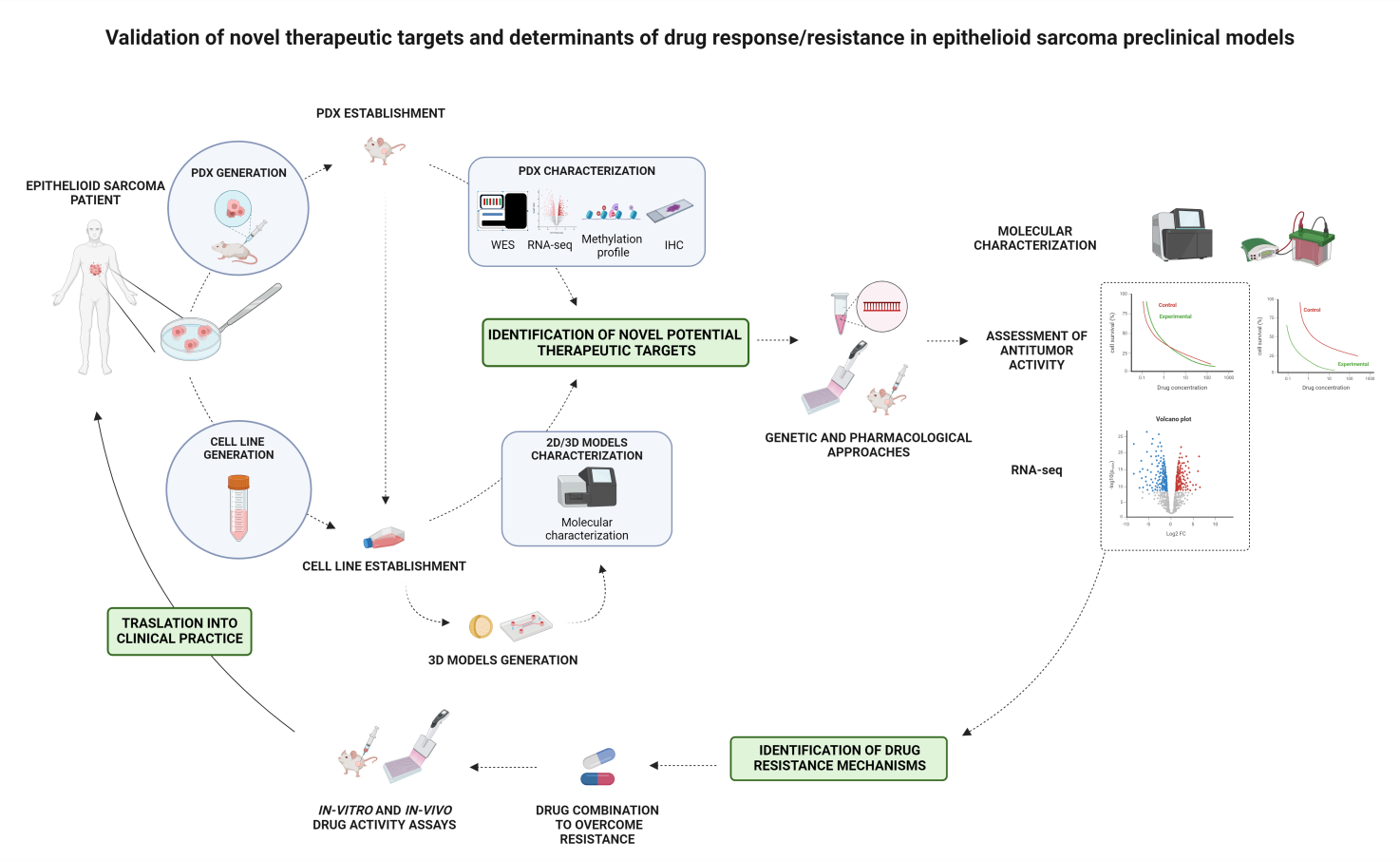
Validation of novel therapeutic targets and determinants of drug response/resistance in epithelioid sarcoma preclinical models
Epithelioid sarcoma (ES) is an ultra-rare and aggressive soft tissue sarcoma characterized by a high tendency for recurrence, metastasis, and poor response to conventional treatments. No standard therapies are available, and prognosis remains poor, especially for patients with metastatic disease. This PhD project focuses on generating and characterizing translatable ES models, such as PDXs and cell lines, to study the disease biology and validate novel therapeutic targets and drug resistance mechanisms. The research aims to identify molecular vulnerabilities and test innovative therapeutic strategies, including drug combinations and epigenetic inhibitors, to expand treatment options for ES patients.
Anna Zanichelli
Unit: Molecular immunology
Director of studies: Dr. Sangaletti Sabina
Supervisors: Dr. Fortunato Orazio, Dr. Verderio Paolo
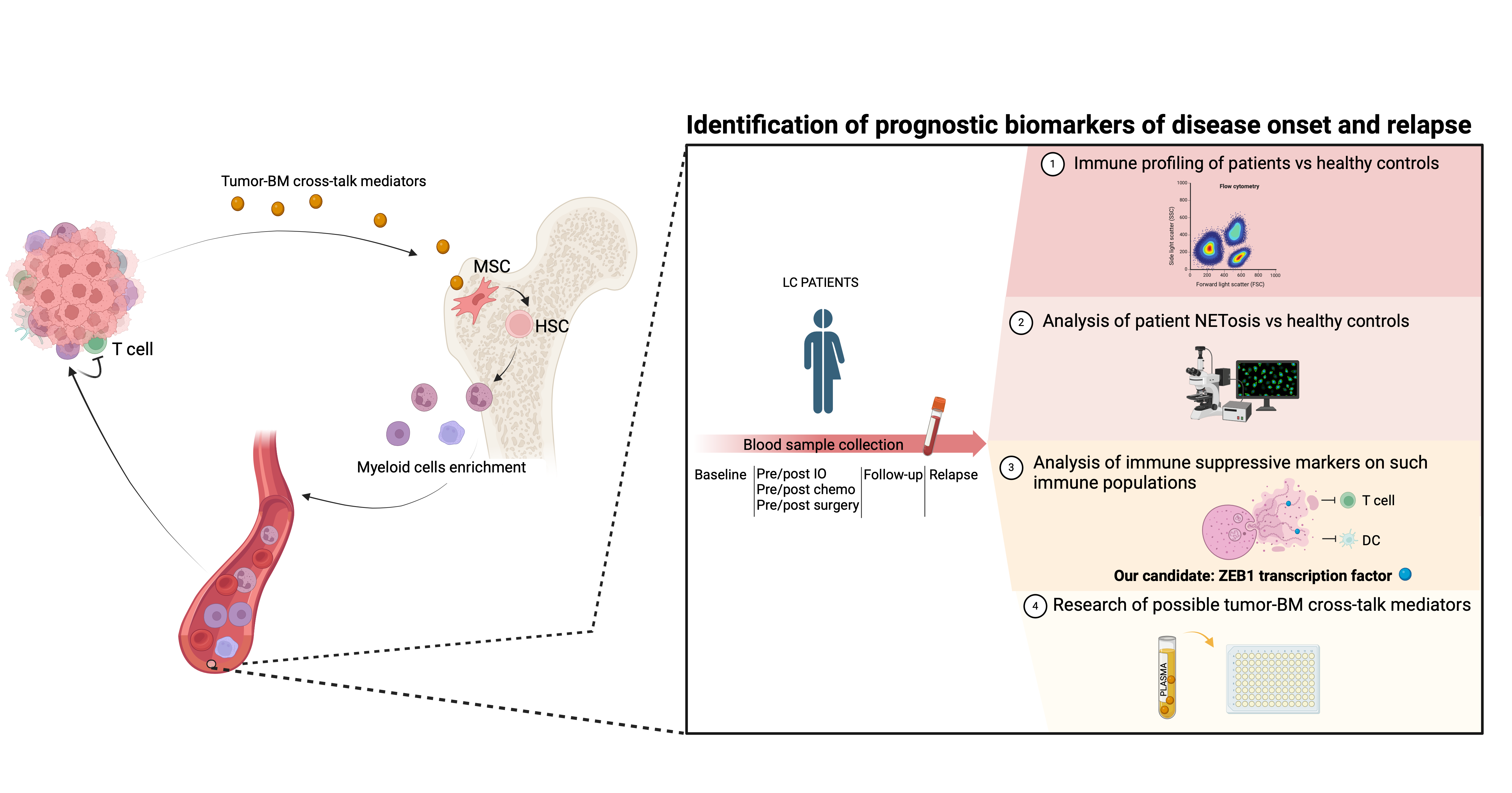
Exploiting the role of neutrophils extracellular traps (NETs) and associated ZEB1 molecule in the development of immune suppressive niches in lung cancer
Despite significant progress in cancer therapies, lung cancer (LC) remains challenging, with low survival rates largely due to late diagnosis or relapses from residual cancer cells. Therefore, early detection of primary tumors or relapse could greatly improve survival outcomes for patients. In the tumor context, immune profiles are often modulated and altered to support tumor initiation, progression, and metastasis. Cancer involves the constant signaling between the tumor and the bone marrow, creating a tumor microenvironment rich in myeloid cells, including neutrophils, monocytes, and immature cell populations, as a result of emergency hematopoiesis. Thus, studying these circulating cells may reveal valuable signals for diagnosing LC. This project mainly aims to validate early myeloid-derived suppressor cells (eMDSCs) and neutrophils as predictive biomarkers for lung cancer onset and relapse. Additionally, it explores the role of the ZEB1 transcription factor in immune suppression, focusing on its collocation along the neutrophil extracellular traps (NETs) through studies in both human and mice models. Putative mediators in the signaling between the tumor and the bone marrow are also investigated.